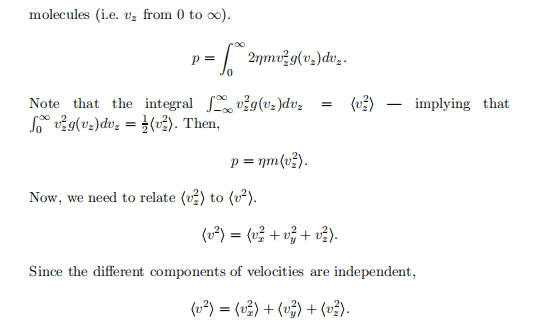
This course is a real-time, online course where the instructor and students meet via web conferencing tools, at scheduled days and times. Instructors and students share information, ideas and learning experiences in a virtual course environment. Participation in synchronous courses requires students to have reliable, high-speed internet access, a computer (ideally with a webcam), and a headset with a microphone.
Let $Q_{B} Q_{R}, T_{R}$ and $W$, respectively, denote, the heat drawn by the refrigerator from the body at temperature $T$, out of which $Q_{R}$ is rejected in the room at temperature $T_{R}$ and a work $W$ is performed on the refrigerator. Now,
$$
Q_{R}=Q_{B}+W \text { and } Q_{B}=Q_{R}-W
$$
And the Coefficient of performance of the refrigerator, $(\mathrm{COF})=\frac{W}{Q_{R}}=\frac{\left(T_{R}-T\right)}{T_{R}}$ or
$$
Q_{R}=\frac{W T_{R}}{\left(T_{R}-T\right)}
$$
Substituting this value of $Q_{R}$ in Eq. S-7.10.1 gives:
$$
\begin{aligned}
&Q_{B}=Q_{R}-W=\frac{W T_{R}}{\left(T_{R}-T\right)}-W=W\left(\frac{T}{\left(T_{R}-T\right)}\right) \
&W=\left(\frac{\left(T_{R}-T\right)}{T}\right) Q_{B}
\end{aligned}
$$
shows that in order to draw a quantity of heat $Q_{B}$ from the body at temperature $T$ the amount of work required to be done is $W$ whichis equal to $\left(\frac{\left(T_{R}-T\right)}{T}\right) Q_{B}$.
PHS2061 COURSE NOTES :
The thermal capacity (at constant volume) is defined as $C_{V} \equiv\left(\frac{\partial U}{\partial T}\right){V}$, which in the present case is $K T^{3}$. Using the relation $\left(\frac{\partial S}{\partial T}\right){V}=\frac{1}{T}\left(\frac{\partial U}{\partial T}\right){V}=\frac{K T^{3}}{T}=K T^{2}$, and hence, $$ \Delta S=\int{T_{R}}^{0} K T^{2} d T=-\frac{K}{3} T_{R}^{3}
$$
Negative sign indicates that this is the decrease in the entropy.
Change in internal energy of the body may be calculated using the relation
$$
d U=C d T \quad \text { or } \quad \Delta U=\int_{T_{R}}^{0} K T^{3} d T=-\frac{K}{4} T_{R}^{4}
$$